2017
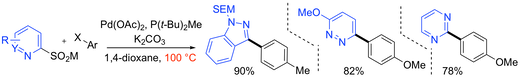
“Catalyst selection facilitates the use of heterocyclic sulfinates as general nucleophilic coupling partners in palladium-catalyzed coupling reactions”, Tim Markovic, Benjamin N. Rocke, David C. Blakemore, Vincent Mascitti and Michael C. Willis, Org. Lett. 2017, 19, 6033–6035. (doi: 10.1021/acs.orglett.7b02944). See our correction: doi: 10.1021/acs.orglett.8b01120).
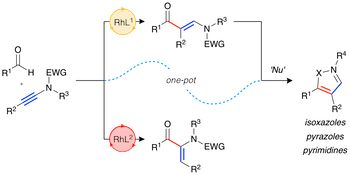
“Exploiting rhodium-catalysed ynamide hydroacylation as a platform for divergent heterocycle synthesis”, Robert N. Straker, Manjeet K. Majhail and Michael C. Willis, Chem. Sci. 2017, 8, 7963-7968. (doi: 10.1039/C7SC03795C)
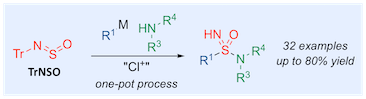
“One-pot, three-component sulfonimidamide synthesis exploiting the sulfinylamine reagent N-sulfinyltritylamine, TrNSO”, Thomas Q. Davies, Adrian Hall, and Michael C. Willis, Angew. Chemie. Int. Ed. 2017, 56, 14937–1494.1(doi: 10.1002/anie.20170859)
“A C-H cyanation of 6-ring N-containing heteroaromatics”, Bryony L. Elbert, Alistair J. M. Farley, Timothy W. Gorman, Tarn C. Johnson, Christophe Genicot, Bénédicte Lallemand, Patrick Pasau, Jakub Flasz, José L. Castro, Malcolm MacCoss, Robert S. Paton, Christopher J. Schofield, Martin D. Smith, Michael C. Willis, and Darren J. Dixon, Chem. Eur. J. 2017, 23, 14733–14737. (doi. 10.1002/chem.201703931)

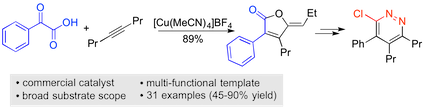
“A copper(I)-catalyzed addition/annulation sequence for the two-component synthesis of γ-ylidenebutenolides”, Sangwon Seo and Michael C. Willis, Org. Lett. 2017, 19, 4556–4559. (doi: 10.1021/acs.orglett.7b02151)
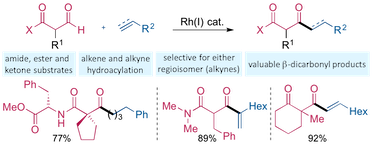
“Exploiting carbonyl groups to control intermolecular rhodium-catalyzed alkene and alkyne hydroacylation”, Thomas J. Coxon, Maitane Fernández, James Barwick-Silk, Alasdair I. McKay, Louisa E. Britton, Andrew S. Weller and Michael C. Willis, J. Am. Chem. Soc. 2017, 139, 10142–10149. (doi: 10.1021/jacs.7b05713)
“Enantioselective three-component assembly of beta’-aryl-enones using a rhodium-catalyzed alkyne hydroacylation/aryl boronic acid conjugate addition sequence”, Ming Gao and Michael C. Willis, Org. Lett. 2017, 19, 2734–2737. (doi: 10.1021/acs.orglett.7b01087)

“Pyridine sulfinates as general nucleophilic coupling partners in palladium-catalyzed cross-coupling reactions with aryl halides”, Tim Markovic, Benjamin N. Rocke, David C. Blakemore, Vincent Mascitti and Michael C. Willis, Chem. Sci. 2017, 8, 4437-4442. (doi: 10.1039/C7SC00675F)

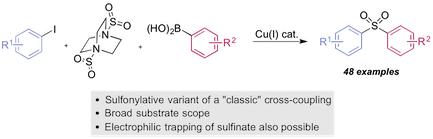
“Copper(I)-catalyzed sulfonylative Suzuki-Miyaura cross-coupling”, Yiding Chen and Michael C. Willis, Chem. Sci. 2017, 8, 3249–3253. (doi: 10.1039/c6sc05483h)
“Toolbox study for application of hydrogen peroxide as a versatile, safe and industrially-relevant green oxidant in continuous flow mode ”, Benjamin Martin,* Joerg Sedelmeier, Anaïs Bouisseau, Patricia Fernandez-Rodriguez, Julien Haber, Florian Kleinbeck, Sonja Kamptmann, Flavien Susanne, Pascale Hoehn, Marian Lanz, Laurent Pellegatti, Francesco Venturoni, Jeremy Robertson, Michael C. Willis and Berthold Schenkel, Green Chem. 2017, 19, 1439–1448. (doi: 10.1039/C6GC02899C)
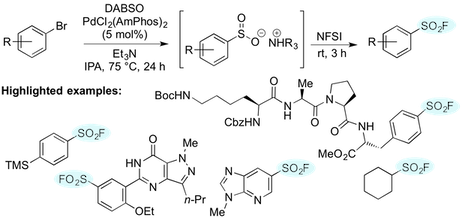
“One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides”, Alyn T. Davies, John M. Curto, Scott W. Bagley, and Michael C. Willis, Chem. Sci. 2017, 8, 1233-1237. (doi: 10.1039/C6SC03924C
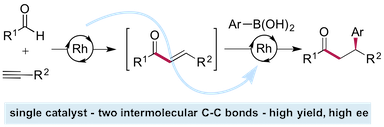
“Sequential catalysis: Exploiting a single rhodium(I) catalyst to promote an alkyne hydroacylation–aryl boronic acid conjugate addition sequence” Maitane Fernández, Matthias Castaing and Michael C. Willis, Chem. Sci. 2017, 8, 536-540. (doi. 10.1039/C6SC03066A)